

(An exception to this rule is a reversible or "isentropic" process, such as frictionless adiabatic compression.) Processes that decrease total entropy of the universe are impossible.
Thus, while a system can undergo some physical process that decreases its own entropy, the entropy of the universe (which includes the system and its surroundings) must increase overall. The formulation of the second law that refers to entropy directly is as follows: In a system, a process that occurs will tend to increase the total entropy of the universe. Thus, the theorems of thermodynamics can be proved using any form of the second law and third law There are many ways of stating the second law of thermodynamics, but all are equivalent in the sense that each form of the second law logically implies every other form.

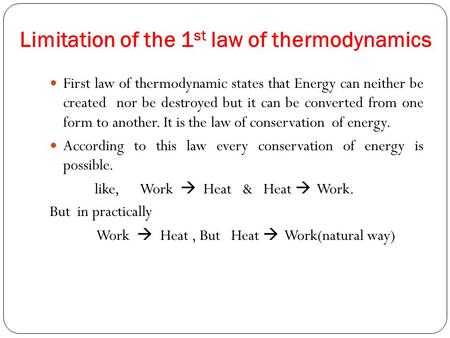
There are many versions of the second law, but they all have the same effect, which is to explain the phenomenon of irreversibility in nature. Entropy is a measure of how far along this evening-out process has progressed. In simple terms, the second law is an expression of the fact that over time, ignoring the effects of self-gravity, differences in temperature, pressure, and density tend to even out in a physical system that is isolated from the outside world. The second law traces its origin to French physicist Sadi Carnot's 1824 paper " Reflections on the Motive Power of Fire", which presented the view that motive power ( work) is due to the fall of caloric ( heat) from a hot to cold body ( working substance). The second law of thermodynamics is an expression of the universal law of increasing entropy, stating that the entropy of an isolated system which is not in equilibrium will tend to increase over time, approaching a maximum value at equilibrium.


 0 kommentar(er)
0 kommentar(er)
